Personalised cancer treatments – and why they’re important
24 April 2024
Professor Jayesh Desai is a Medical Oncologist and clinician researcher at Peter Mac.
He and his team are working to develop what are known as “precision” therapies for rare cancers – particularly sarcomas – hoping to give cancer patients a better chance of survival.
We spoke to him about his work.
Q: Let’s start with sarcoma. What and how rare is it?
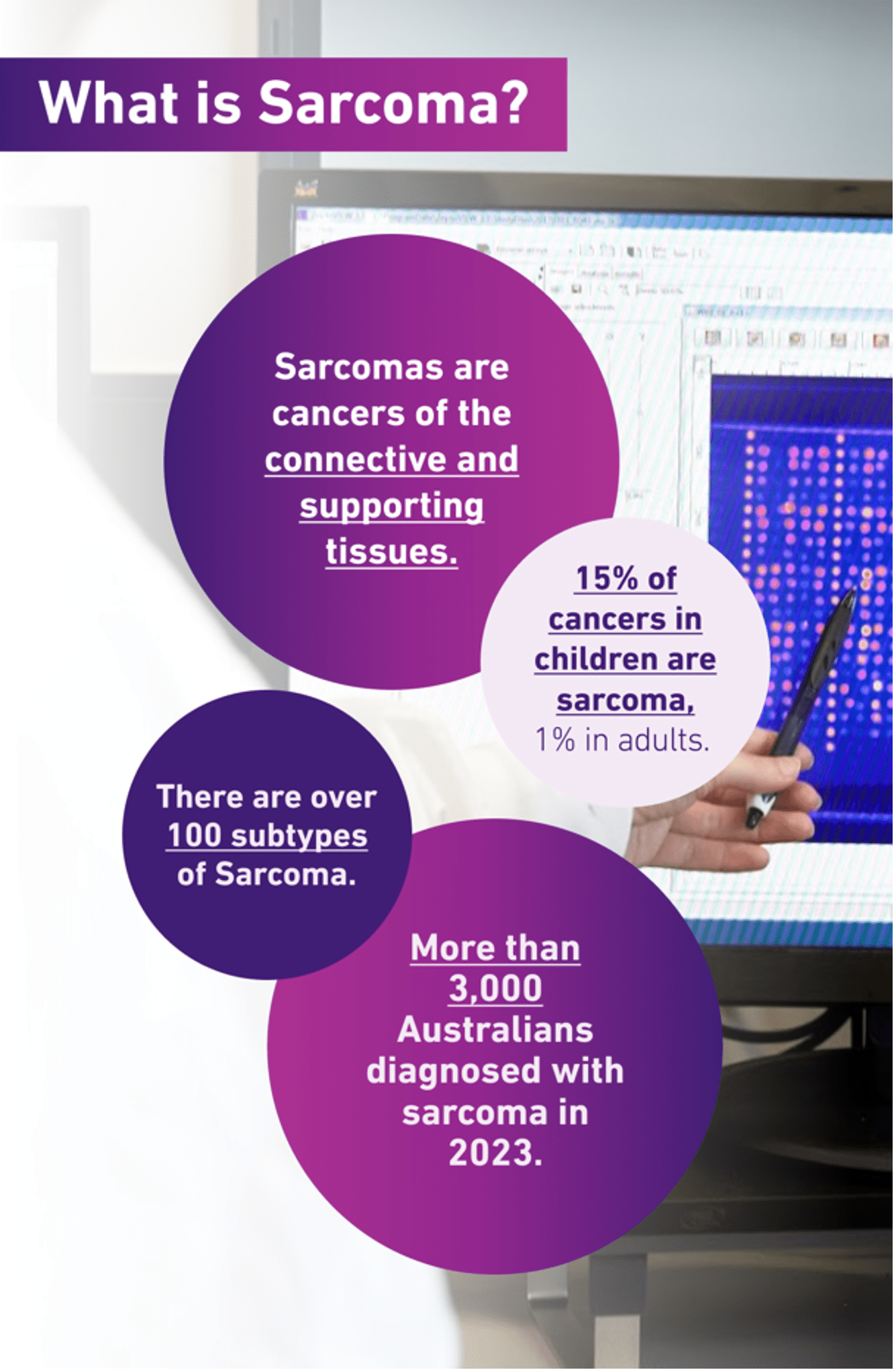
Sarcomas are cancers of the connective and supporting tissues in the body – the things that hold your body together.
About 3,000 people were diagnosed with sarcoma in Australia in 2023.
But there are more than 100 subtypes of sarcoma, many of which are very rare, and can be rarer still because of the different locations and tissue types within the body in which they can occur.
Sarcomas are also less common in adults – they make up only about 1% of adult cancers – compared with 15% in children.
Q: Why are rare cancers like sarcoma such a challenge for researchers?
A: It comes down to case numbers.
In the cancer space, there are what are loosely called rare cancers. We can’t apply the same research principles as we have done for cancers like breast cancer and bowel cancer and so on, because we don't have the ability to develop large research studies or research trials, at least at a local level, because the numbers are too small .
And sarcomas are one of the leading rare cancers from that perspective.
For example, at Peter Mac we might be treating a particular sarcoma subtype, where we may only see a few patients a year.
Q: You’ve focused on sarcoma for over 20 years. How have things changed?
A: I would describe the changes in cancer research for sarcoma in recent years, broadly, as one of continuing incremental progress.
But in some subtypes, they've been quite profound. And when we've made those profound gains, they have been because we've recognised and understood what makes those particular subtypes unique.
It’s critically important to put a lot of effort into recognising what particular subtype of sarcoma a particular patient has.
We can therefore now be more specific and tailored about the therapy we might want to use in that patient, and take a highly customised approach. That’s really important.
Q: That brings us rather nicely onto personalised treatments, one of the key areas you’re working on. What are they and why are they important?
A: At their simplest, they’re treatment plans that are developed for a particular person with their particular type of cancer in mind.
The ambition is to increase the impact of treatment, making it more likely to result in a cure, and often reducing the number of life-altering side-effects a patient might experience.
They’re important because they help save lives.
What’s more, this work is also highly applicable to many other cancer types, so it comes with the potential to save even more lives.
Broadly speaking, cancers are such complex diseases, and the only way we are going to have an impact is by continuing to innovate.
This highly targeted approach is one of the key areas of innovation at the moment.
Q: What’s the secret to finding these new personalised treatments?
A: Expert pathology input is the absolute key.
There are over 100 subtypes of sarcoma. The area of research where there’s been the greatest progress in treating sarcoma is in the instances where there’s been a really good, in-depth understanding of the unique features that exist within certain subtypes.
The devil is in the detail.
Q: And are there any special techniques you’re using to get that pathology?
A: Absolutely. There’s a very exciting new frontier that’s recently opened in the field of genomics and genomic testing.
Genomics is the study of the whole genome – all of the genes in an individual. When you apply it to cancer research it’s the business of looking at a patient’s full genetic code, looking for markers that treatments might be able to target.
When you look at cancers from way down at the gene level, it can help to remove some of the guesswork from cancer treatment.
It lets us match together an in-depth understanding of an individual patient's cancer with the molecular drivers that are underlying it – and identify therapies that are more suitably matched towards them.
Q: You call it a new frontier?
A: Sure. Genomics itself isn’t exactly new, but it’s application in cancer research and now cancer care for an individual patient is.
As an area of study generally, it began in the 1970s, with the human genome first being sequenced in the early 2000s.
That took over 10 years to complete at a cost of over $3 billion.
But in the past decade, there’s been an absolute step-change in the practical application of genomic testing.
Today, the key elements of a human genome can be sequenced in matter of days at a cost in the hundreds of dollars, not in the billions, making it a very viable and desirable diagnostic tool, particularly for cancers.
Q: Are you using genomics to find more targeted sarcoma treatments?
A: It’s definitely helping us get a better understanding of patients’ sarcomas and what's underlying them, and whether we can identify more focused therapies towards it.
The breakthroughs are going to come from continuing to break down sarcomas into their biological subtypes through genomics.
But, of course, more research is needed and the pool of case numbers is still very low.
Q: How are you getting around that?
A: As I mentioned earlier, because these types of cancers are so rare, we don't have the ability to develop large research studies or research trials at a local level.
So, what we've learned to do is to work together as a team, not just within Australia but with other international centres of excellence in cancer research, including with sarcomas. And this helps bring the best opportunities to our patients.
We might only get three cases in Australia in a year, but if you then multiply that by 50, from all of the other similar centres that we work with around the world, we can develop a much deeper understanding of that disease, and gain those key insights in that were just too challenging 10 years ago.
Q: Is this international collaboration one of the highlights of your role?
A: It’s certainly one of them.
Peter Mac is a fantastic place where we can bring together outstanding clinical care and expertise with research. That goes from fundamental research, to really understand what goes on with cancer, to how we apply that in individual patients through clinical trials.
This is the ideal place to bring together all of those things and discover new cures. And that’s an absolute highlight.
Q: What support do researchers like you need? Is there any way readers can help?
All cancer researchers at Peter Mac, myself included, need access to vital resources to conduct their research.
Genomics is just one tool, but there are so many other core technologies, as well as cutting-edge equipment and great research thinking, that we need access to.
When people donate to cancer research, it goes to fund those resources. So if they give a donation, that’s directly helping the work of people like me, helping accelerate our work, and delivering new treatments and cures for all kinds of cancers.
Want to show your support for people like Professor Desai? Donate to life-saving cancer research today.
@Follow us on Instagram (@SupportPeterMac)










